In vitro tests for reproductive toxicity
What is the objective of toxicity tests?
Avantea uses a battery of in vitro tests for reproductive toxicity able of revealing adverse effects of chemicals and/or medical devices on specific reproductive processes. The tests are based on the application of assisted reproductive technologies, such as oocyte in vitro maturation, in vitro fertilization and early embryo development. Some of the applications include:
- To test disposable labware and laboratory items and media that are used to recover and culture human gametes and embryos
- To test human assisted reproduction devices and products that come into contact with the reproductive tract
- To develop grouping and read-across methods to screen chemicals, pharmaceuticals and cosmetic ingredients for potential human reproductive toxicity

Why the bovine model?
Key human reproductive processes such as ovarian folliculogenesis, oocyte maturation and preimplantation embryo development are more closely related to bovine than to laboratory rodents. Bovine in vitro tests are based on protocols that mirror those used in assisted reproduction and are therefore particularly suitable to reveal toxic effects. No animal sacrifice is needed because bovine oocytes can be collected in large numbers from animals already destined to enter the food chain and bovine semen is commercially available as frozen product.
The types of toxicity tests available
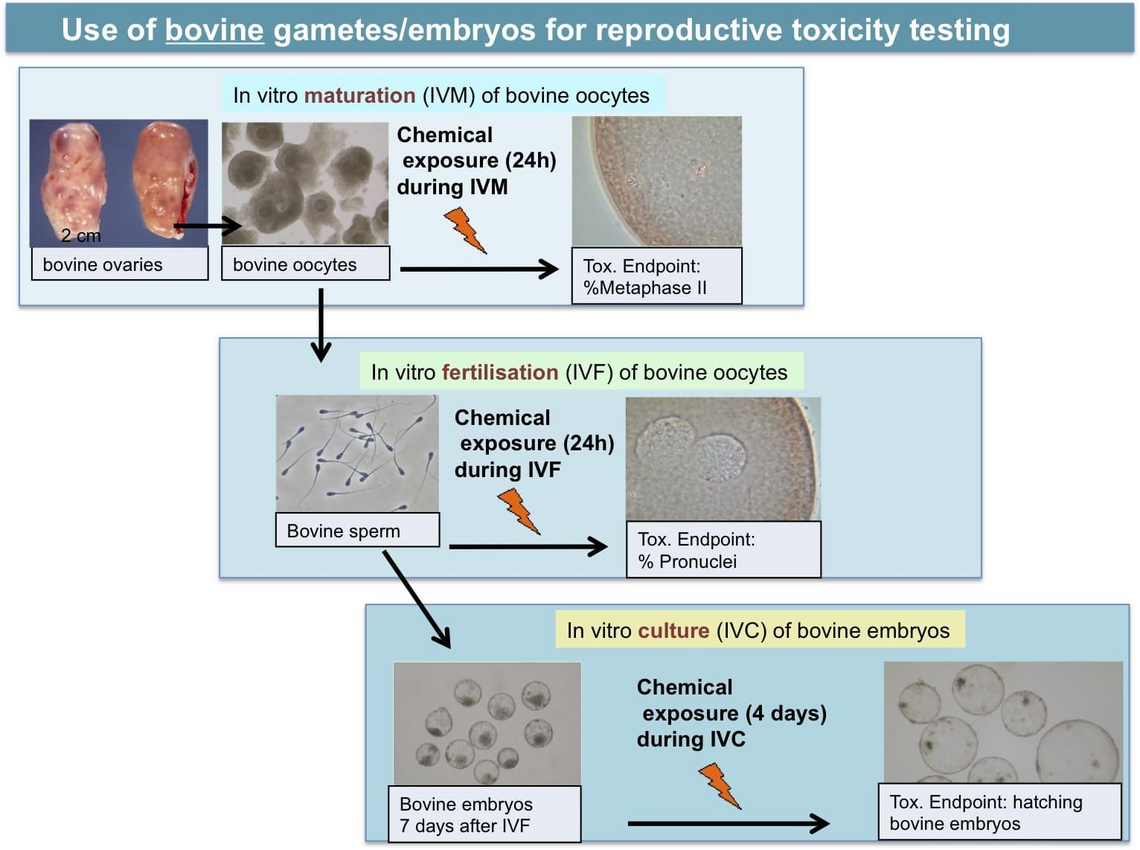
In vitro maturation test (IVM): it is a test which riproduces in vitro the oocyte maturation process. It corresponds to the in vivo pre-ovulatory phase and it allows to detect specific toxic effect on the oocyte.

In vitro fertilization test (IVF): this test involves the exposure of oocytes and spermatozoa to substances to be tested and it allows to evaluate the toxic effect both on the oocytes and on the oocyte-spermatozoa interaction during fertilization.

In vitro embryo culture test (IVC): this test is based on the use of bovine embryos one week after fertilization in order to evaluate toxic effect on the embryo development up to the hatched blastocyst stage, mimicking the beginning of the embryo development up to implantation.

Citoxicity test on 3T3 fibroblast cell line (Alamar blue assay): it allows to evaluate aspecific, non reproductive, toxic effects on mammalian cell proliferation and viability in vitro. This test has to be used in association wih IVM, IVF and IVC tests to differentiate generic toxicity from specific effect on reproductive processes.

In accordance with the 3Rs principle, in order to apply these assays it is not necessary to sacrifice animals as the oocytes are collected from ovaries of cows which have already been allocated in the food chain, whereas the bovine sperm is readly available on the market as frozen.
For more information on in vitro toxicity tests or to request a quote, contact us.